Learning Objective
Manufacturers of medicines and healthcare products must establish, validate and maintain an equipment cleaning programme. This is a regulatory requirement because validated cleaning procedures contribute to the assurance of product purity and safety. This module provides a comprehensive account of equipment cleaning validation requirements and procedures. It follows the work of a pharmaceutical company's validation team as they establish and validate the cleaning program for a new production line.
Who will benefit from this module?
Manufacturing personnel in the pharma/biotech, dietary supplement, and medical devices industries need to understand the principles and practice of cleaning validation, as set out in this module. In particular, the module provides essential learning for engineering, production, and quality management personnel in the pharmaceutical industry.
Learning Objectives
- Define cleaning validation terminology, and explain regulatory requirements
- Determine the scope of cleaning validation
- Carry out and validate tests of cleanliness
- Determine acceptance criteria
- Develop and execute a cleaning validation protocol
- Analyse and report cleaning validation results, and outline an ongoing cleaning and monitoring programme
Module Outline
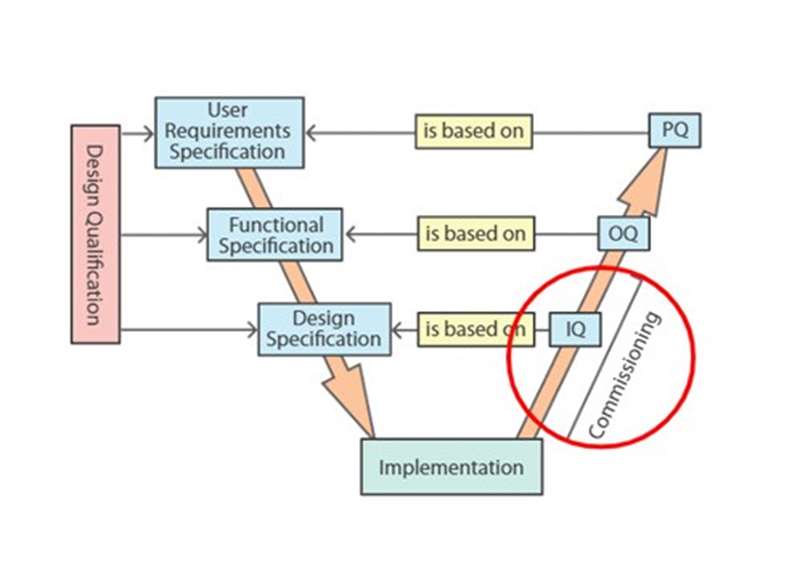
Introduction - An introduction to the validation project that provides a case study for Zenosis modules on validation, plus definitions and a glossary.
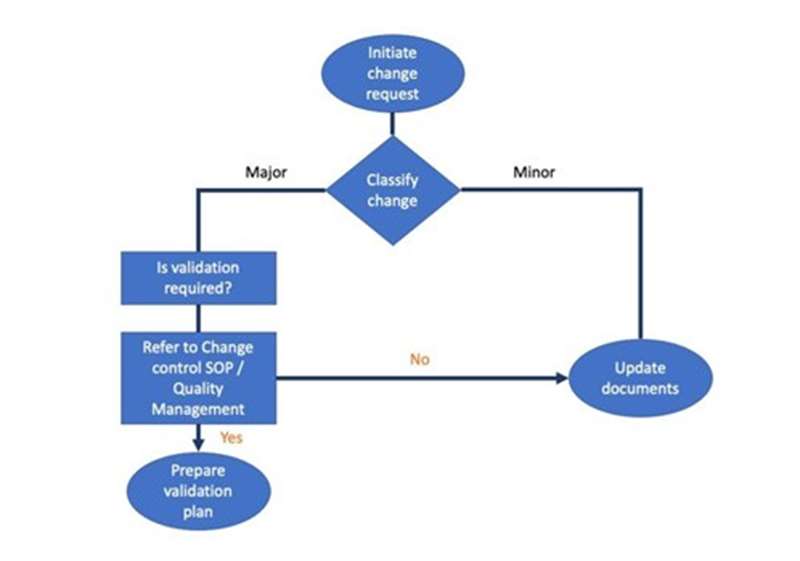
Scope of cleaning validation - This session compares and contrasts different approaches to equipment cleaning validation: equipment-train vs individual item; common vs dedicated equipment; batch-to-batch vs product-to-product cleaning; automated vs manual. It identifies requirements that cleaning SOPs need to meet. It explains the bracketing of products for cleaning validation. It outlines the qualification of clean-in-place systems, and the use of previous cleaning validation data. Finally, it describes techniques for testing surface residues.
Validation of test methods - This session identifies analytical techniques that are appropriate for cleaning validation studies. It defines limit of detection (LOD) and limit of quantitation (LOQ). Finally, it explains how to carry out swab recovery studies.
Acceptance criteria - This session identifies factors to be considered when determining acceptance limits for product carryover. It explains how to use toxicity and solubility data in determining acceptance criteria. It describes the selection of worst-case, follow-on, and representative products for cleaning validation studies. It explains how to calculate Maximum Allowable Carryover (MAC), and Surface Area Limit (SAL) for swab testing.
Writing and executing protocols and writing reports - This session identifies essential elements of a cleaning validation protocol. It describes how to specify worst-case conditions for cleaning validation, and sampling at the most difficult-to-clean locations. It explains why a cleaning validation protocol is usually applied to actual batch manufacture. It provides checklists for preparedness to execute a cleaning validation protocol, and for the documentation of results. It summarises the content of a cleaning validation report. Finally, it outlines ongoing cleaning requirements after validation.
Assessment - The assessment tests the learner’s assimilation of the module’s content.