Learning Objective
Good Clinical Practice (GCP) inspections and audits are carried out to provide assurance that: the rights, safety and welfare of clinical trial subjects are protected; the data that constitute the results of the trials are accurate and reliable; and the trials are carried out in compliance with relevant legal requirements.
This module describes what investigational medicinal product sponsors, contract research organisations and clinical investigators can expect when they undergo inspection or audit. It focuses in particular on inspection by European and US regulators.
The module describes general principles of GCP inspection and audit, discusses preparation for an inspection, and sets out in detail what European and US FDA inspectors will examine. Finally it describes post-inspection actions by the regulator and the inspected party.
Who will benefit from this module?
This module will benefit all those involved in clinical research who already understand the basics of GCP. It will be of value to staff working in clinical, medical and QA departments of pharmaceutical companies and CROs, to independent clinical research associates, and to healthcare professionals conducting clinical studies.
Learning Objectives
- Discuss principles of GCP inspections and audits
- Specify activities to be carried out in preparation for an inspection
- Describe what happens when a European regulator inspects the site of a sponsor or contract research organisation
- Describe what happens when a European regulator inspects the site of a clinical investigator
- Describe what happens when the US Food and Drug Administration inspects the site of a sponsor or contract research organisation
- Describe what happens when the US Food and Drug Administration inspects the site of a clinical investigator
- Specify post-inspection actions by the regulator and the inspected party
Module Outline
Module overview - An outline of the module’s scope and objectives, and notes on terminology.
Principles of GCP inspections and audits - Principles, applicable in any regulatory jurisdiction, of inspections and audits: their purpose, who carries them out, in what circumstances, and their possible consequences; routine versus targeted inspections; system versus study-specific inspections.
Preparing for an inspection - Actions you can take to prepare your site for a GCP inspection, whether you work for a sponsor or CRO or as a clinical investigator.
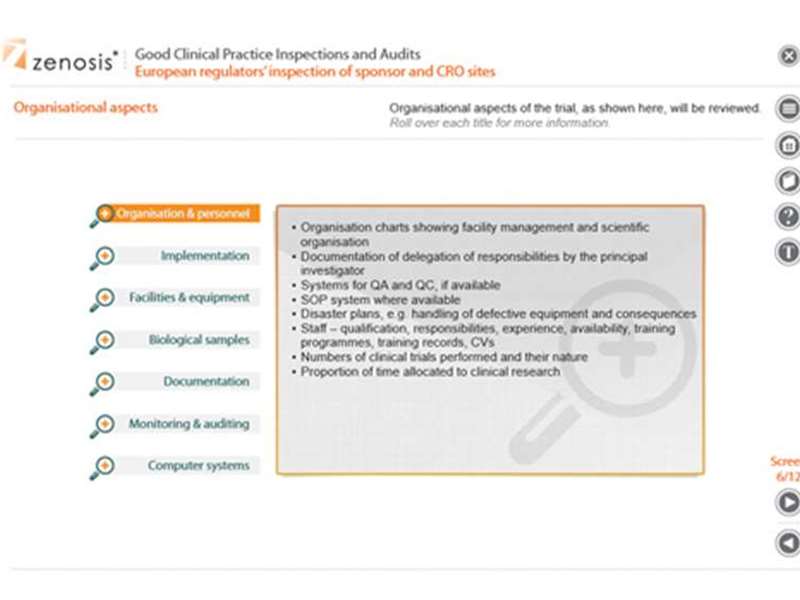
European regulators’ inspection of sponsor and CRO sites - Procedure for inspection of the site of a sponsor or CRO by the regulatory authority of a member state of the European Economic Area: pre-inspection provision of an inspection request and plan to the inspectee; quality system inspection; study-specific inspection.
European regulators’ inspection of investigator sites - Inspection of legal and administrative aspects, organisational aspects, informed consent provisions, subject data, and management of investigational medicinal products.
FDA inspection of sponsor and CRO sites - An outline of pre-inspection activity among the relevant FDA offices is followed by detailed description of what the inspectors examine as regards organisation and personnel, study registration, selection and monitoring of investigators, study monitoring, quality assurance, safety and adverse event reporting, data collection and handling, record retention, financial disclosure, computer systems, electronic records and signatures, and investigational product.
FDA inspection of investigator sites - An outline of investigators’ legal obligations and the possible scope of an inspection is followed by detailed description of what the inspectors examine as regards authority and administration, clinical protocol, institutional review board, informed consent, source documents, CRFs, financial disclosure, investigational product control, records retention, reports to sponsor, and monitoring.
Action after an inspection or audit - This session describes post-inspection actions by regulators, and responses by inspected parties, with particular reference to European and US regulators: meetings at the close of inspections, inspection reports, classification of findings, responses and action plans, post-inspectional correspondence, and possible consequences of serious deficiencies.
Assessment - Multiple-choice mastery assessment.