Learning Objective
Validation of equipment, services, systems and processes is vitally important in the medicines and healthcare products industries. Regulatory authorities require documented evidence that manufacturing processes will consistently result in products meeting predetermined quality standards. This module provides an introduction to validation and to the regulations and guidance that apply to it. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
Who will benefit from this module?
Manufacturing personnel in the pharma/biotech, dietary supplement, and medical devices industries need to understand the principles and practice of validation, as set out in this module. In particular, the module provides essential learning for engineering, production, and quality management personnel in the pharmaceutical industry.
Learning Objectives
- Define terms relating to validation
- Access sources of regulations and guidance on validation in the medicines and healthcare products industries
- Specify the phases of equipment qualification and process validation and describe the goals of each phase
- Use risk assessment to determine the scope of a validation project
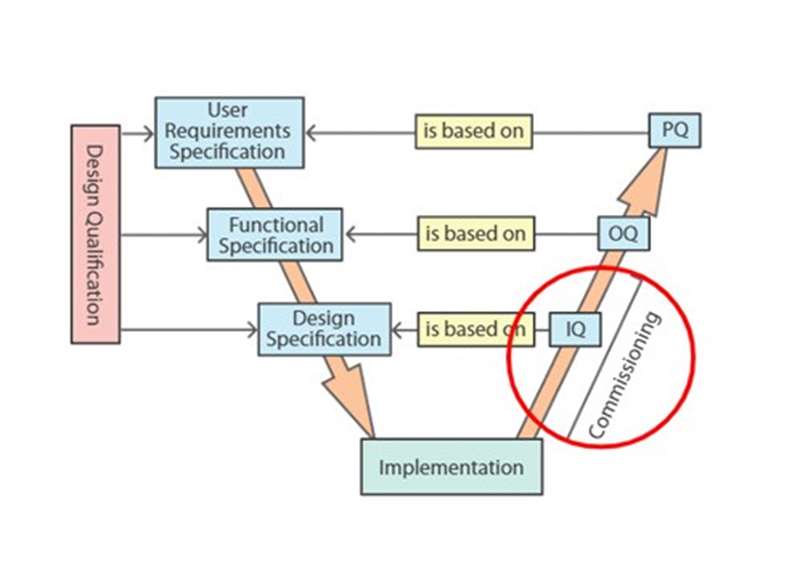
- Describe the relationships between specifications and protocols in the V model of validation
- Discuss criteria for User Requirements Specification, Factory Acceptance Testing, and Site Acceptance Testing
- Identify important documents created and used during a validation project, and specify their relationships
- Describe procedures for change control of validation documentation
Module Outline
Regulations, guidance and definitions - This session emphasises the need to comply with regulatory requirements and guidance on validation, identifying important regulatory authorities and international collaborations. It identifies the phases of equipment qualification, describes the purpose of process validation in relation to process control, and defines important terms relevant to validation.
Development of risk-based approach to validation - This session explains why, in the absence of process validation, testing of samples is inadequate to provide assurance of product quality, safety and effectiveness. It outlines the historical development of validation requirements and identifies some current trends. It emphasises the importance of a risk-based approach to validation and describes factors for assessing risk.
What to validate? - This session explains how to develop the scope of a validation plan, distinguishing critical and non-critical equipment, services and utilities. It specifies criteria for validation of computerised systems and for selection of process steps for validation. Finally, it discusses the importance of validation of cleaning and laboratory test methods.
Stages of validation - This session describes the ‘V model’ approach to equipment qualification. It outlines the contents of a User Requirements Specification document, and explains the role of Factory Acceptance Testing and Site Acceptance Testing. It distinguishes commissioning and qualification, and describes the phases of qualification and validation. Finally, it identifies standard operating procedures that are created during qualification and validation.
Documentation and change control - This session identifies important documents created during a validation project, and outlines relationships among protocols and reports. It describes how to record deviations and failures, and their resolution. Finally, it discusses requirements for change control of validation documentation.
Assessment - The assessment tests the learner’s assimilation of the module’s content.